August 16, 2013 — In December, 2008, clinicians identified an adverse event at the Veterans Administration Medical Center (VAMC) in Murfreesboro (TN) that sparked a national discussion about the quality and oversight of health care, infection control, and the safe and effective use of reusable medical equipment.[1-5]
This adverse event prompted the Veterans Health Administration (VHA)—which oversees this VAMC and other VA medical facilities—to publish a patient safety alert on December 22, 2008.[2]
Placing emphasis on the importance of training and quality assurance, this safety alert, among other instructions, enjoined directors to perform audits of their respective VA medical facility and to notify the VHA if they identified any breaches similar to the Murfreesboro VAMC’s.[2]
Attention: This is the first in a series of three articles on the topic of irrigation during GI endoscopy. The reader is also directed to the other two:
- 2nd article in series: “Disposable” Irrigation Tubing Used During Gastrointestinal Endoscopy” — click here;
- 3rd article in series: “Guidance for the Safe Use of “Disposable” Irrigation Tubing Used During GI Endoscopy” — click here.

After performing a risk assessment, the VHA on February 9, 2009, notified 6,387 patients who underwent colonoscopy at the Murfreesboro VAMC of this adverse event.[1]
On this same day, the VHA also issued a directive that provided VA medical facilities with procedures to design and implement “a systematic approach” for the proper setup, use, and reprocessing of reusable medical equipment.[3]
Four months later, on June 16, 2009, the VA’s Office of Inspector General (VA-OIG) published a comprehensive, if also fault-finding, report that—based on a number of recently confirmed infection-control breaches including those of the Murfreesboro VAMC’s—found a number of VA medical facilities have been lax and not complying with directives about the reprocessing of flexible endoscopes, resulting in “a risk of infectious disease to veterans.”[1]
Similar infection-control breaches were also contemporaneously confirmed at VA medical facilities in the Caribbean, including the VAMC in Puerto Rico.
Read Dr. Muscarella’s related article about these breaches in Puerto Rico: “Potentially Faulty Assessment by the Veterans Health Administration of the Risk of Infection Associated with a Number of Confirmed Breaches.“
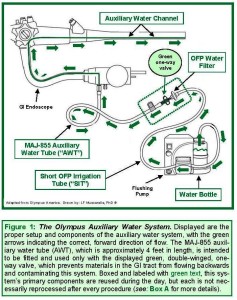
Figure 1 (right) displays the proper setup of the Olympus auxiliary water system,[1] which is used to irrigate the GI tract’s mucosa during GI endoscopy. Read a letter by Olympus, dated March 3, 2003, entitled “GI Endoscopes with Auxiliary Water Features.”
The Murfreesboro VAMC’s Errors
An adverse event whose cause apparently had not been previously reported, clinicians noticed blood in the GI endoscope’s accompanying auxiliary water system (Olympus America) while performing colonoscopy at the Murfreesboro VAMC.[1]
The primary function of this auxiliary water system is to irrigate the GI tract’s mucosa during GI endoscopy. An investigation determined that for as many as 5 years, from 2004 until December, 1, 2008, when this breach was detected, this VAMC had been misusing and improperly reprocessing some of this auxiliary water system’s reusable components.[1]
Read Dr. Muscarella’s instructions for reprocessing the MAJ-855 auxiliary water tube and the GI endoscope’s auxiliary water channel.
More specifically, one of this auxiliary water system’s components — the Olympus MAJ-855 Auxiliary Water Tube, referred to herein as the “AWT”[1]— is intended to be fitted and used exclusively with a green, double-winged, one-way valve, whose purpose is to ensure the uni-directional flow of water (or, for example, saline, either of which is usually sterile) during irrigation, from the water bottle into the GI tract (via the GI endoscope’s auxiliary water channel).
Figure 1 (above) displays the Olympus auxiliary water system, with the AWT properly fitted with this valve. This figure’s green arrows indicate the correct direction of the irrigant’s flow.
Note: Although it focuses on the auxiliary water system used with those models of GI endoscopes by Olympus America that feature an auxiliary water channel, this article’s discussions may also be applicable to similar water systems used with the GI endoscopes of other manufacturers, including Pentax and FujiFilm. Nevertheless, this article’s discussion is limited to those irrigation systems that connect to the GI endoscope’s auxiliary water channel.
Entitled “Proper Setup of the Auxiliary Water System,” Box A — click here to read Box A in the more complete PDF version of this article, which is entitled “Faulty Use of a GI Endoscope’s Auxiliary Water Channel” — discusses this auxiliary water system, its proper use and setup, and the reprocessing requirements of its individual components, as applicable.
The AWT’s one-way valve is critical because (the Olympus) GI endoscope’s auxiliary water channel does not itself feature an internal valve to prevent the backflow of potentially infectious materials and fluids.
Instead of using the AWT as intended by its manufacturer, with the requisite one-way valve fitted firmly in place, however, the Murfreesboro VAMC unwittingly modified this water tube, accidentally fitting it with a similar looking—but an incorrect—green, single-winged two-way connector (that is designed to be used with the 10″-long Olympus MH-974 washing tube—not the approximately 4-foot long Olympus MAJ-855 AWT).[1]
The improper fitting of the AWT with this wrong two-way connector—this error is displayed in Figure 2, which is only available in the complete PDF version of this article, which can be downloaded by clicking here)—unintentionally facilitated that which the AWT’s one-way valve is specifically intended to prevent:
the backflow of potentially infectious materials and fluids (see: the black arrows in Figure 2), from the patient’s GI tract (which was, at times, at a higher pressure) into the auxiliary water system (due to it being, at times, at a lower relative pressure) via the GI endoscope’s auxiliary water channel.[1,2]
As a result of this mix-up, blood flowed retrogradely into the auxiliary water system, posing an increased risk of exposing 6,387 affected patients to blood-borne pathogens, including HIV and the hepatitis B (HBV) and C viruses (HCV).[1,2]
That this mix-up, which is displayed in Figure 2, could also have exposed patients to potentially infectious agents other than viruses, such as resistant bacteria, is a possibility that the VHA (soundly or not) dismissed. (Note: Figure 2 is only available in the complete PDF version of this article, which can be downloaded and viewed by clicking here.)
Other breaches in Murfreesboro
The investigation of this error also determined that the Murfreesboro VAMC had been reprocessing the AWT only once at the end of the day—not after each patient procedure as would be especially required of an AWT that had been improperly fitted with a two-way connector.
Discussed in Box A – which is only available in the more complete PDF version of this article entitled “Faulty Use of a GI Endoscope’s Auxiliary Water Channel” – the AWT is reusable and required by its labeling to be reprocessed after each use.[1]
Reprocessing is not indicated for every one of the auxiliary water system’s reusable components, however. Some, like the Olympus OFP (water) Filter, which is displayed in Figure 1, are labeled instead to be replaced and discarded daily.[1] This article labels these types of components that reused without being reprocessed as reposable, or single-day, devices.
The definitions of a reposable device, along with a reusable and disposable, or single-use, device, are provided in Box B (which is embedded, below) for clarity. (None of the auxiliary water system’s components are labeled as single-use items.)
Read Dr. Muscarella’s related article, “Tap Water Used for Irrigation during GI Endoscopy: A Recommendation and Assessment of the Infection Risk.“
A third breach, investigators also determined that the Murfreesboro VAMC had been reusing from one day to the next both the Olympus OFP Filter and the Short Olympus OFP Irrigation Tube (referred to herein and in Figure 1 [see above] as the “SIT”), instead of having discarded both at the end of the day as their labeling requires.

Each of the Murfreesboro VAMC’s confirmed infection-control breaches is listed Box C (which is only available by clicking here on p. 5 of the more complete version of this article in PDF format entitled “Faulty Use of a GI Endoscope’s Auxiliary Water Channel”).[3]
Miami VAMC’s Errors
In response to the VHA’s aforementioned patient safety alert (dated December 22, 2008),[2] staff at the VAMC in Miami (FL) performed an audit and, two weeks later, reported on January 5, 2009, that the auxiliary water system’s AWT was fitted with the correct one-way valve (as displayed in Figure 1; see above), without, however, necessarily investigating the possibility of another related reprocessing breach or deviation being performed.[1]
Attention: Read Dr. Muscarella’s related article about a jury that found a hospital to be “negligent” for improperly cleaning and disinfecting the auxiliary water channel of GI endoscopes.
One month later, on February 4, 2009, the VHA issued a memorandum to all VA medical facilities requesting, among other tasks, that each evaluate the competency of its endoscope-reprocessing personnel.[1] (The requirements specified in this memorandum were formalized into the VHA’s directive, dated February 9, 2009.[3])
In response to this memorandum (and directive), the Miami VAMC performed a second audit in early March, 2009, and this time it identified two breaches similar to the Murfreesboro VAMC’s documented errors.[1]
Namely, according to the VA-OIG’s report of June, 2009, staff of the Miami VAMC found during this second audit that, although it was fitted with the correct one-way valve, the auxiliary water system’s AWT, for as many as 5 years, had not been reprocessed after each use as required by its labeling, having instead been only flushed or rinsed with (sterile) water.[1]
Further, staff determined that clinicians had often not been priming the GI endoscope’s auxiliary water system prior to colonoscopy, instead connecting the AWT to the colonoscope after the procedure was already in progress “in approximately half” of the procedures.[1]
The VHA concluded that, coupled together, these breaches posed a “significant” risk of transmitting diseases and, as a result, it notified 3,260 patients of the potential for their exposure to viruses.[1]
Also during this second audit, staff found that, since May, 2004, the Miami VAMC, like the Murfreesboro VAMC, had not been discarding and replacing daily the auxiliary water system’s SIT (i.e., the short Olympus OFP Irrigation Tube) or the OFP Filter (see: Figure 1, above).[1]
And, in late March, 2009, the Miami VAMC identified “debris” in the auxiliary water channel of “reprocessed” colonoscopes.[1] Box C lists each of the Miami VAMC’s confirmed infection-control breaches.
Disposable Irrigation Tubing Sets
The breaches identified at both the Murfreesboro VAMC and Miami VAMC — listed in Box C (click here to read this box on p. 5 of the more complete version of this article entitled “Faulty Use of a GI Endoscope’s Auxiliary Water Channel“), these breaches were assessed by the VHA to pose a clinically significant risk of infection that warranted patient notification[1] — not only bring into focus the importance to patient safety of an otherwise obscure “one-way valve,” but also raise for discussion whether an alternative system—a “disposable” tubing set for irrigation of the GI tract via the GI endoscope’s auxiliary water channel—might be easier to use and reduce the risk of patient harm.
The VA-OIG report of June, 2009,1 which can be read by clicking here, focused on the reprocessing practices not only of the Murfreesboro VAMC and the Miami VAMC, but also of the VAMC in Augusta (GA). According to this report, the Augusta VAMC determined in November, 2008, that it had been improperly reprocessing flexible laryngoscopes for almost a year.
Click the link to read two articles (article #1 and article #2) by Dr. Muscarella that provides more details about the Augusta VAMC’s breach.[5] Table 2 of the article at this link lists this Augusta VAMC’s specific breaches.
Consideration that use of a disposable irrigation tubing set might reduce the likelihood of a medical facility misusing or improperly reprocessing a component of a reusable auxiliary water system is all the more apt, because if any one factor likely contributed (at least in part) to the breaches confirmed at the Murfreesboro VAMC and Miami VAMC, it might be confusion arising from the disparate reprocessing instructions associated with the individual components of the Olympus (or another manufacturer’s comparable) auxiliary water system.
(Note: As mentioned in the VA-OIG’s report of June, 2009,[1] two days after the VHA issued its aforementioned directive,[3] dated February 9, 2009, a manufacturer’s representative visited the Miami VAMC to recommend replacement of the Olympus auxiliary water system with a disposable irrigation tubing set advertised as comparable.)
“Different reprocessing instructions for components of the auxiliary water subsystem ‘creates confusion’.”1 — The VA-OIG, in its aforementioned report of June, 2009.
In brief, the individual components of the Olympus auxiliary water system are associated with three different sets of reprocessing requirements:[1]
- the OFP Filter and SIT (and single-day water bottles) are reposable (see: Box B, above, for the definition of “reposable,” requiring daily replacement without reprocessing);
- the AWT and the GI endoscope’s auxiliary water channel are reusable, requiring reprocessing after each procedure; and
- a reusable water bottle, which requires reprocessing, but only once at the end of the day (not after each procedure).
Attention: Click here to read Box A and Box C in the more complete PDF version of this article, which is entitled “Faulty Use of a GI Endoscope’s Auxiliary Water Channel.“
Without adequate training of staff, such dissimilar reprocessing instructions may cause confusion and, possibly, the reusable device’s misuse, posing the potential for an increased risk of disease transmission.
What’s Next
In summary, this article discusses the misuse and improper reprocessing of an auxiliary water system by the Murfreesboro VAMC and Miami VAMC, which resulted in the notification of thousands of affected patients of the potential for infection.
The VHA and others have published recommendations and actions to prevent such reprocessing breaches, including the instruction to ensure staff are trained and to perform audits routinely.[2,4,5]
Whether disposable tubing sets might be easier to use and less prone to contamination and disease transmission than reusable auxiliary water systems warrants discussion. — Lawrence F Muscarella PhD
Another action—consideration of the appropriateness of using “disposable” tubing sets (for irrigation of the GI tract’s mucosa via the GI endoscope’s auxiliary water channel) in lieu of the Olympus auxiliary water system[4] (some of whose components, as this article points out in Box A, require reprocessing)—is discussed in the second and final article in this series, which is featured in this newsletter’s next issue.
Among other topics, this sequel discusses the features, designs, labeling, marketing, and common use of these disposable irrigation tubing sets.
Remember: Click here to read Box A in the more complete PDF version of this article, which is entitled “Faulty Use of a GI Endoscope’s Auxiliary Water Channel.“
(Note: This article’s discussion applies to the use of disposable tubing sets for the indicated purpose of irrigation of the GI tract via the GI endoscope’s auxiliary water channel, not necessarily of irrigation via a flexible endoscope’s working, or biopsy, channel.)
Additional suggested reading
- “Disposable Irrigation Tubing Sets used during Gastrointestinal (GI) Endoscopy.” (Click here to read this article.)
- “Recommendations to Prevent Healthcare-Acquired Infections.” (Click here to read this article)
- “The Risk of Infection Associated with Gastrointestinal Endoscopy.” (Click here to read this article.)
- “Case Study: A Contaminated Gastrointestinal Endoscope.” (Click here to read this article.)
References
- VA Office of Inspector General. Healthcare Inspection: Use and Reprocessing of Flexible Fiberoptic Endoscopes at VA Medical Facilities. Report No. 09-01784-146. June 16, 2009. (pp. 1-45)
- Veterans Health Administration (VHA). Improper Setup and Reprocessing of Flexible Endoscope Tubing and Accessories. Patient Safety Alert AL09-07. December 22, 2008. (pp. 1-7)
- Department of Veterans Affairs. VHA Directive. 2009-004. February 9, 2009. Use and Reprocessing of Reusable Medical Equipment (RME) in Veterans Health Administration Facilities. (pp. 1-5)
- Muscarella LF. Recommendations to Prevent HAIs. The Q-Net Monthly 2011 Jan-Apr;17(1-4):1-8.
- Muscarella LF. Patient Safety Concerns in Puerto Rico. The Q-Net Monthly 2010 Apr-May-Jun;16(4-6):7-11.
Article by: Lawrence F Muscarella, PhD, posted 2-22-2013; updated 2/3/2016, Rev A.